What is HOCl - Hypochlorous Acid
What is Hypochlorous Acid (HOCl)? What is it for and what does it do?
HOCl is the scientific formula of Hypochlorous Acid, a weak acid with strength of a light citrus juice. HOCl is naturally produced by white blood cells in all mammals to fight bacteria and viruses, protect and heal the body.
HOCl is a strong oxidant that acts against invading bacteria, fungi and viruses. In the 1970s, the first HOCl was produced by passing electricity through the brine solution. HOCl is the only viable disinfectant that can be used against pathogens including Tuberculosis, Legionella, H1N1 (swine flu), M. Chelonea, Poliovirus, HIV, Aureus, E. Coli, Candida Albicans, Enterococcus Faecalis, P Aeruginosa, including CORONA VIRUS because it is the most effective substance against the pathogenic microorganisms in nature since it is produced by the defense system of the human body against bacteria and viruses.
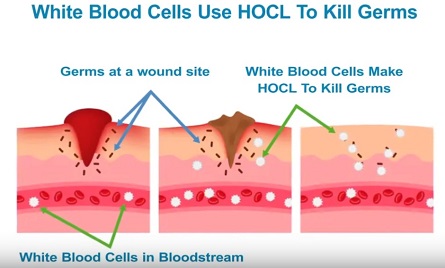
Cells called white blood cells in our body produce a physiological substance called Hypochlorous Acid (HOCl) while fighting against bacteria and viruses. This substance has the largest spectrum and it is themost effective biocide known in nature. Thanks to its weak acidic nature, it is neither irritating or harmful to human health. Therefore, it is the best antiviral and antibacterial disinfectant known in nature moreover the human body itself produces the best disinfectant.
When the human body is subjected to any microbial attack, 1 Neutrophil produces 0.1 Um Hypochlorous Acid, and this amount has been scientifically proven to kill 15 million coli bacilli within 1 minute.
How is HOCl - Hypochlorous Acid produced?
ECA is short form of Electro Chemical Activation.
HOCl production from electrochemical activation (ECA) of brine was developed in the 1970s.
ECA is a patented technology that produces hypochlorous acid (HOCL) or super oxidized water, which is a 100% nature-soluble, disinfectant, by electrolysis in the electrochemical cell only from salt and water. (Our brand "SuperOx" is the short for super oxide water.)
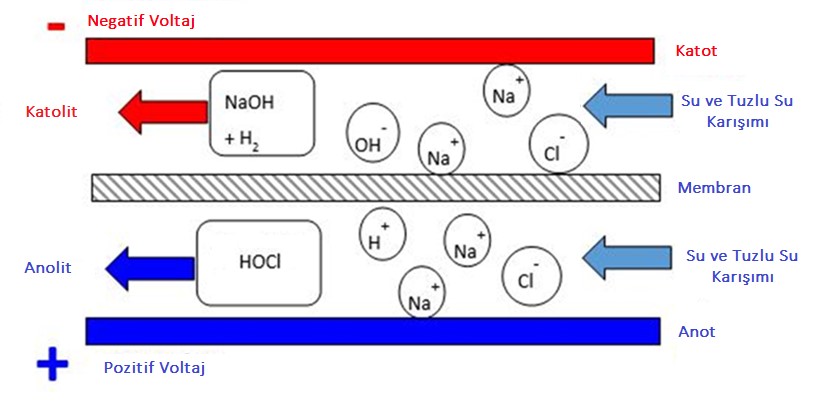
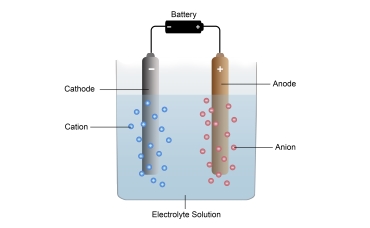
Solutions collected on the cathode side are called Catholyte, and solutions collected on the anode side are called Anolyte. Anolyte solution consists mainly of water and Hypochlorous Acid.
Substances formed in anolyte solution as a result of electrolysis;
Properties of Hypochlorous Acid solution;
Why HOCl is more effective in killing pathogenes according to other disinfectants?
Comparison of Hypochlorous Acid and Sodium Hypochlorite (bleach);
Hypochlorous Acid has a neutral electrical charge while the hypochlorite ion carries a negative electrical charge. Hypochlorous acid acts very quickly and oxidizes the bacteria within seconds, and the hypochlorite ion needs to wait half an hour to do so.
Cell surfaces of microorganisms carry a negative electrical charge that causes the negatively charged hypochlorite ion to be repelled from the cell surface, making the hypochlorite ion less effective in killing germs. The fact that hypochlorous acid has a neutral electric charge allows it to penetrate the protective barriers surrounding the microorganisms more effectively.
The ratio of the two solutions is determined by the relative acidity (pH) of the water. Many scientific publications have shown that the optimum pH level of HOCl in human application is 6.2. Therefore, the pH level of the disinfectants we produce under the brand SuperOX is 6.2.
Hypochlorous Acid is a strong oxidizer and has been proven by many scientific publications that it is 100 times more effective than Sodium Hypochlorite (bleach) in killing microbial pathogens.
Hypochlorous acid is naturally produced by the white blood cells of all mammals. It plays an important role in the immune system, which kills pathogens through oxidation and chlorination.
Hypochlorous acid is a physiological compound that the human body produces against bacteria and viruses. Unlike negatively charged hypochlorite (OCl-), this molecule is neutrally charged, meaning it is uncharged, unique in this respect. So why is this important?
Disinfectants and microbial pathogens interact with each other in a manner similar to magnets. If you put together two negatively charged magnets, they repel each other. Bacteria and hypochlorite are both negatively charged and act like two negatively charged magnets that repel each other. Hypochlorous acid (HOCl) is neutrally charged and is not repelled by bacteria. HOCl easily penetrates the walls of bacteria and destroys the cell membrane of bacteria and viruses by its strong oxidation potential.
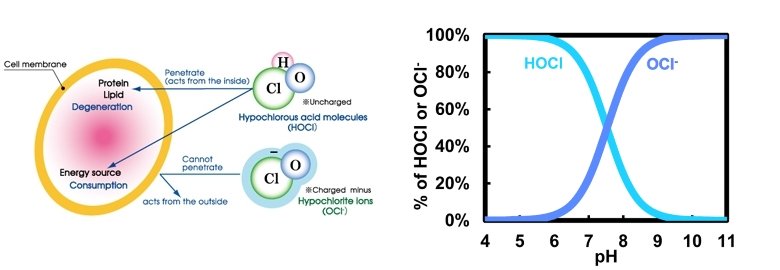
Why PH is this much important?
A free chlorine (FAC) molecule is an unbound molecule. There are three forms of free chlorine: chlorine gas, Hypochlorous Acid and hypochlorite. Assuming a constant temperature of 25 ° C, when the pH is below 3, free chlorine will be present in the solution as chlorine gas. When the pH is above 7.5, over 50% of free chlorine will be hypochhorite ion (OCl-), and as pH rises to 11, all of the free chlorine in the environment will turn into hypochlorite. Free chlorine solution hypochlorous acid (HOCl) will dominate between pH 3 and pH 7.5.
The ideal pH is 6.2. All of the molecules that act as active disinfectants here are HOCl. The pH of the human skin is 5.5, and the pH just below the skin is 7.0. Our skin has a pH of 5.5 to act as a shield against bacteria and viruses, meaning it is slightly acidic.
ALCOHOL-based disinfectants used, if used very often, create cracks in the skin and these cracks open the way to the new body for bacteria and viruses. Therefore, extreme caution should be exercised when using alcohol-based disinfectants.

Hypoclorous Acid (HOCI) is a healthy safe economic natural environmental solution.

HOCI (Hypoclorous Acid) Physiology
Hypochlorous acid is one of the most effective biocides known. Its chemical structure is HOCl. It is produced by the immune system to kill invasive organisms and fight infection. White blood cells produce and release this natural oxidant to fight invading pathogens.
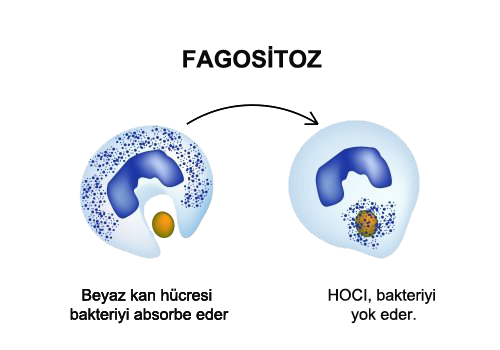
If a wound is formed on the skin, it creates a passage for the harmful pathogens to invade our cells. Neutrophils, a type of white blood cell, travel where blood pathogens invade. After cells are invaded, the hypochlorous acid responds in milliseconds and destroys the pathogens before the body's immune system damages the cell. Invasive pathogens are ingested by white blood cells through a process called phagocytosis. Hypochlorous Acid is a biocide and kills the microbial pathogen within millisecond contact time. This antimicrobial process is called Oxidative Explosion.
HOCI Technology
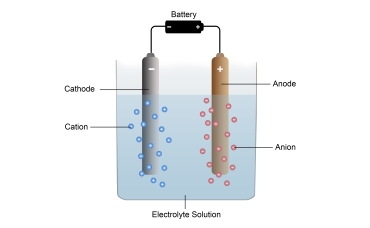
Electrolysis
Electrolysis is the passage of direct electric current through an ionic substance. It was first described by Michael Faraday in the 1830s.
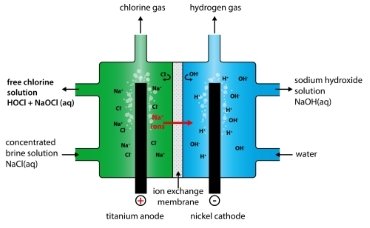
Membrane Electrolysis
Membrane electrolysis produces a strong acidic HOCl and an alkaline by-product from a NaCl solution (table salt in water).
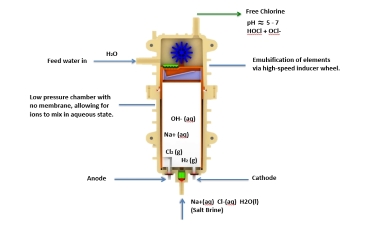
Single Cell Electrolysis
Single cell technology has been developed to produce a more stable HOCl solution at optimum pH, without an alkaline byproduct of NaOH.
Stable Hypoclorous Acid Production
Cell Membrane Technology
The electrolysis cell has two chambers separated by a membrane, an anode chamber and a cathode chamber. The membrane is made of a polymer that only allows positive ions to pass through the cathode compartment. A sodium chloride solution is injected into the anode chamber. Positively charged sodium ions pass from the membrane to the cathode side, but negatively charged chloride ions do not.
Two solutions produced this way: anolyte and catholyte. On the anode side, hypochlorous acid solution with a strong acidic and ORP> 800 mV is produced. On the cathode side, NaOH solution with strong alkali and ORP <-800 is produced
Single Cell Technology
In single cell electrolysis, Hypochlorous acid solution is produced on the anode side. Electrolysis cells have a single chamber containing both anode and cathode and is designed to form a single solution with ORP> 800. The HOCl solution remains stable and HOCl molecules are disabled only when exposed to an organic surface or oxygen in the air.